Plasmid Isolation: Why add SDS?
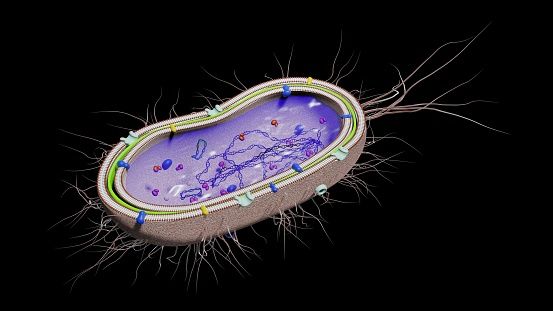
Introduction:
Sodium dodecyl sulfate (SDS) is a vital component in plasmid isolation protocols from Escherichia coli (E. coli). As a powerful detergent, SDS plays a crucial role in lysing bacterial cells and releasing their contents, including plasmid DNA. Let's delve into why SDS is added to the isolation medium, how it facilitates cell lysis, and its importance in maintaining the integrity of the extracted DNA.
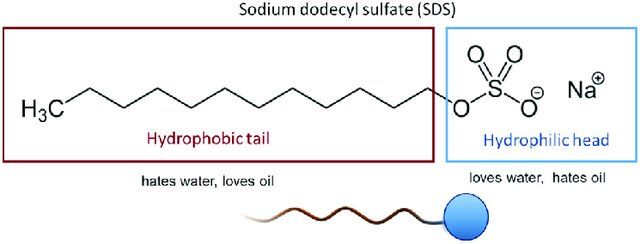
Solubilization of Phospholipids:
E. coli cells are enveloped by a lipid bilayer membrane composed of phospholipids, which act as a barrier protecting the cellular contents. When isolating plasmids, it's essential to disrupt this membrane to access the DNA inside. SDS comes into play as a detergent that solubilizes the phospholipids of the cell membrane. By interacting with the hydrophobic tails of phospholipids, SDS disrupts the lipid bilayer structure, leading to cell lysis and the release of cellular contents, including plasmid DNA.
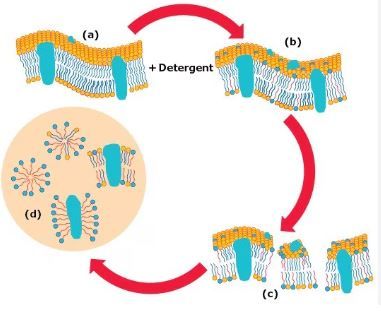
Incubation Time:
While SDS is effective in lysing bacterial cells, it's essential to be mindful of the duration of incubation. Extended exposure to SDS can potentially damage the DNA molecules, including plasmids. Therefore, it's recommended to incubate the solution containing SDS at room temperature for a maximum of 5 minutes. This brief incubation period ensures efficient cell lysis while minimizing the risk of DNA damage.
Denaturation under Alkaline Conditions:
Following the addition of SDS, the isolation medium is typically adjusted to alkaline conditions. Alkaline conditions, often achieved by the addition of a base such as sodium hydroxide (NaOH), denature both plasmid and genomic DNA. This denaturation step is crucial for separating the DNA molecules and preparing them for subsequent purification steps.
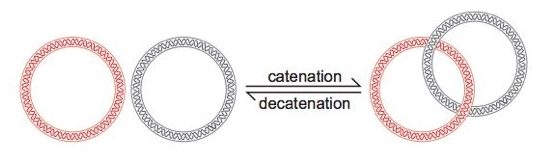
Catenated Structure of Plasmid DNA:
One unique characteristic of plasmid DNA is its catenated structure, wherein the two strands of the plasmid DNA are intertwined together. This intertwined structure enables plasmids to be separated effectively from genomic DNA and other cellular components in the subsequent steps of the isolation process. The denaturation under alkaline conditions helps unravel the catenated plasmid DNA, facilitating its separation and purification because, in the next DNA renaturation step, plasmid DNA find it much easier to renature than the much larger genomic DNA molecule due to their catenated form.
Gentle Mixing to Preserve DNA Integrity:
After the addition of SDS, it's important to handle the solution with care to avoid damaging the DNA molecules, particularly plasmids. Gentle mixing or inversion of the solution helps ensure thorough distribution of SDS while minimizing shear forces that could potentially break DNA strands.
Conclusion:
In summary, SDS serves as a critical component in plasmid isolation from E. coli by facilitating cell lysis and the release of DNA. By solubilizing phospholipids and proteins of the cell membrane, SDS enables access to the cellular contents, including plasmid DNA. However, it's essential to be mindful of the incubation time and handle the solution gently to preserve the integrity of the extracted DNA.
Additionally, alkaline conditions and the catenated structure of plasmid DNA play significant roles in the subsequent steps of the isolation process, ensuring the successful purification of plasmids for downstream applications in molecular biology.