Plasmid Isolation 101: Why add EDTA?
Introduction:
In the realm of molecular biology, preserving the integrity of DNA is crucial, especially when isolating plasmids from bacterial cells like Escherichia coli (E. coli). One key component used in plasmid isolation protocols is ethylenediaminetetraacetic acid, better known as EDTA. EDTA serves an essential role in safeguarding plasmids by inhibiting the action of nucleases, enzymes that can degrade DNA. Let's delve into why EDTA is added to the medium during plasmid isolation.
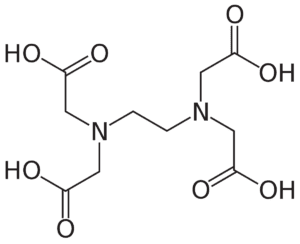
EDTA as a Chelating Agent:
EDTA is a versatile compound widely employed in biochemical and molecular biology experiments. It acts as a chelating agent, meaning it forms stable complexes with metal ions by coordinating multiple bonds with a single metal ion. In the case of plasmid isolation, EDTA's ability to chelate metal ions is particularly advantageous.
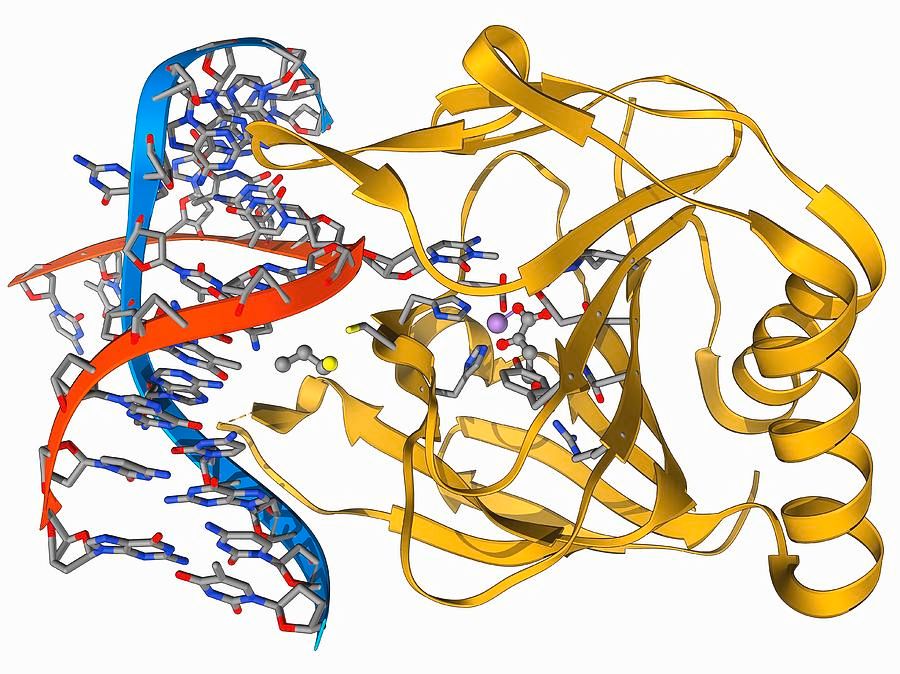
Nucleases and DNA Degradation:
Nucleases are enzymes that catalyze the hydrolysis of phosphodiester bonds within nucleic acids. These enzymes are ubiquitous in biological systems and play essential roles in DNA repair, recombination, and degradation. However, during plasmid isolation, the presence of nucleases poses a significant threat to the integrity of the isolated DNA.
When nucleases encounter unprotected DNA, they can rapidly degrade it, leading to the loss of desired genetic material. This degradation can occur even within a relatively short timeframe, jeopardizing the success of plasmid isolation experiments. Therefore, it is crucial to implement strategies to inhibit nuclease activity and preserve the integrity of the isolated DNA.
EDTA's Protective Role:
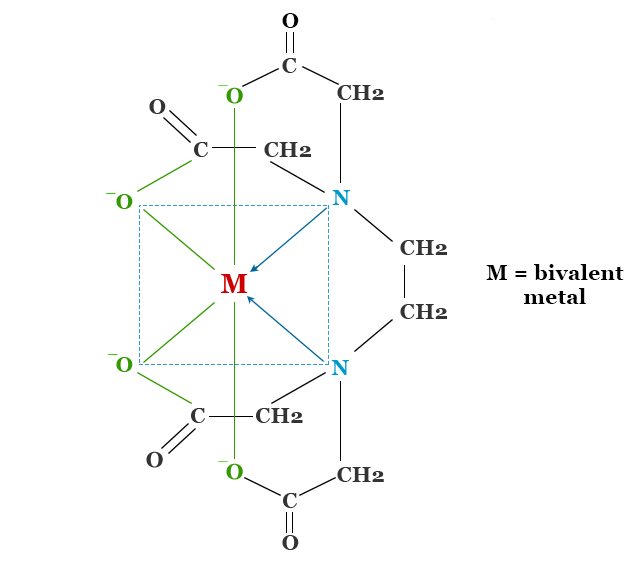
EDTA comes to the rescue by effectively inhibiting nucleases through a simple yet powerful mechanism. By binding to divalent metal cations, such as magnesium (Mg2+) and calcium (Ca2+), EDTA prevents these ions from serving as cofactors for nuclease activity. Nucleases rely on these metal ions for their catalytic function, and without them, their ability to degrade DNA is significantly impaired.
When EDTA sequesters divalent cations, it creates a chelate complex that effectively shields the DNA from nuclease attack. By depriving nucleases of their essential cofactors, EDTA acts as a potent protector of plasmids during the isolation process.
Incorporating EDTA into the medium during plasmid isolation ensures that the isolated DNA remains intact and free from degradation by nucleases. This preservation of DNA integrity is critical for downstream applications such as cloning, sequencing, and gene expression studies.
Conclusion:
In summary, EDTA serves as a vital component in plasmid isolation protocols by inhibiting the activity of nucleases through chelation of divalent metal ions. By preventing nucleases from degrading DNA, EDTA helps preserve the integrity of the isolated plasmids, ensuring their suitability for further molecular biology experiments. The inclusion of EDTA in the isolation medium underscores its importance in maintaining the quality of genetic material and ultimately contributes to the success of experimental endeavors in molecular biology.