Plasmid Isolation: Why add Lysozyme?
Introduction:
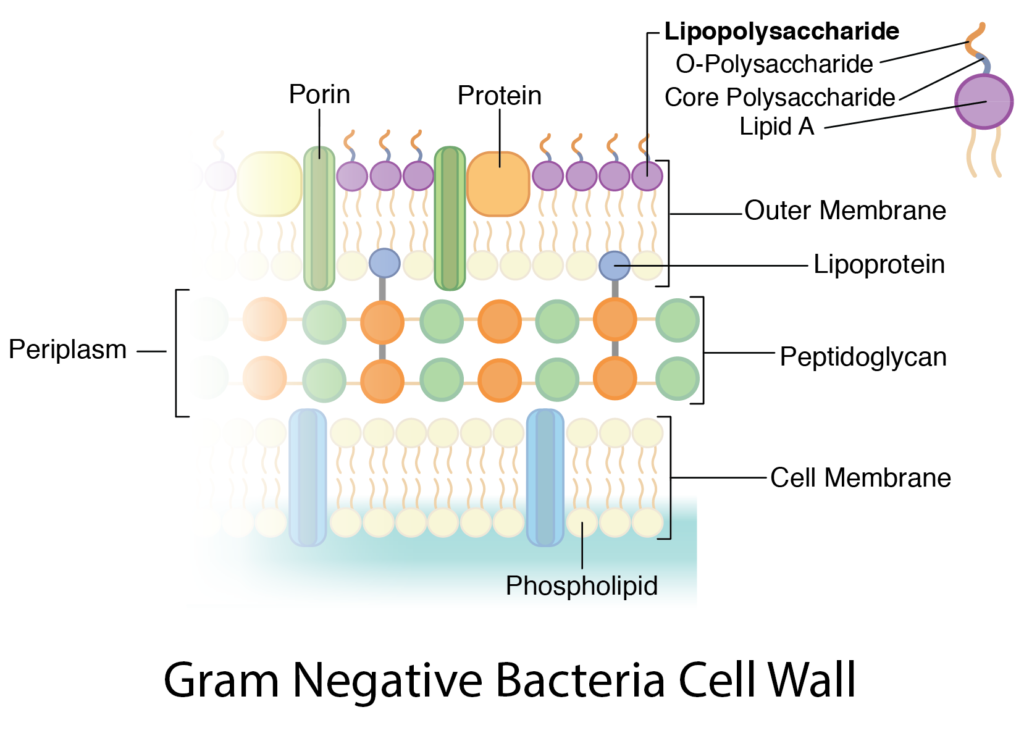
Gram-negative bacteria such as Escherichia coli (E. coli) possess both inner and outer membranes, with a thin peptidoglycan layer in between, serving as their enclosure. When isolating plasmids from E. coli, it's essential to break down these cell walls to access the genetic material; plasmid DNA and genomic DNA within. This is where lysozyme comes into play. Lysozyme, a natural enzyme found in various organisms, serves as a potent tool for lysing bacterial cells during plasmid isolation. Let's explore why lysozyme is added to the isolation medium and how it facilitates the extraction of DNA from E. coli cells.
Bacterial Cell Walls:
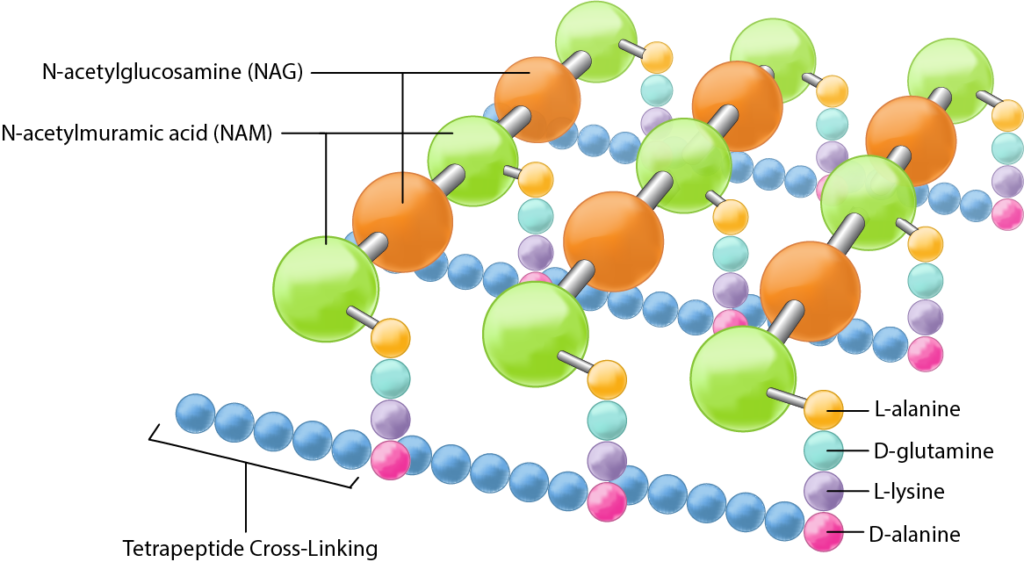
Bacterial cell walls play a crucial role in maintaining cell shape and protecting the cell from environmental stressors. The primary component of bacterial cell walls is peptidoglycan, a polymer made up of alternating sugars (N-acetylglucosamine and N-acetylmuramic acid) cross-linked by short peptides. This structure provides strength and rigidity to the cell wall.
Lysozyme:
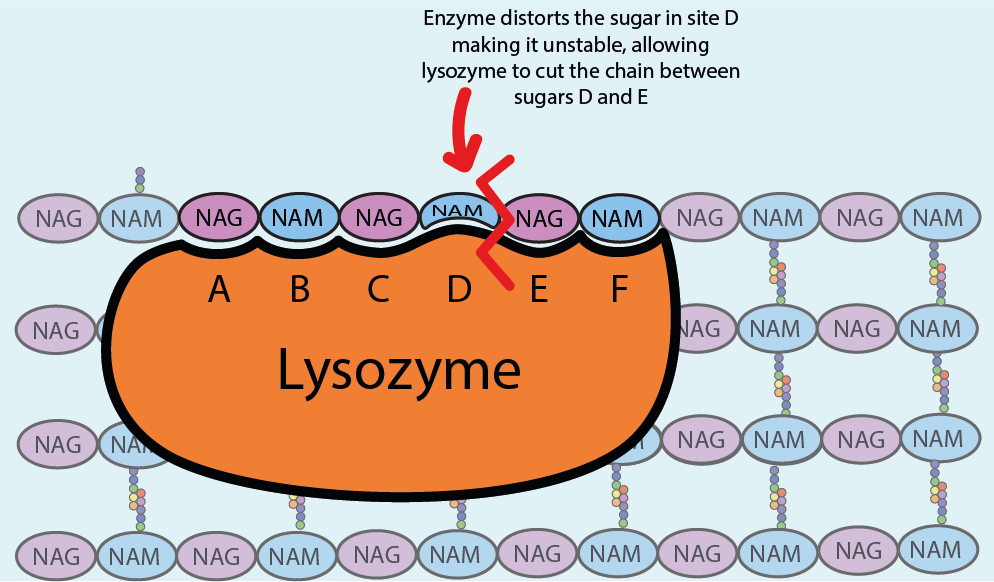
Lysozyme, a natural antibacterial enzyme, targets the integrity of bacterial cell walls by attacking the peptidoglycan layer. Specifically, lysozyme hydrolyzes the glycosidic bonds between the sugar components of peptidoglycan, effectively breaking down the cell wall and leading to cell lysis. It also damages the bacteria triggering the activation of autolytic enzymes within the bacterial cell wall, which can lead to their destruction or death. This enzymatic activity of lysozyme is crucial for releasing the contents of bacterial cells, including plasmids and genomic DNA, during plasmid isolation procedures.
Optimum Temperature for Lysozyme Activity:
Lysozyme exhibits optimal activity at physiological temperatures, with its most effective operating temperature being around 37°C (98.6°F), which coincides with the typical temperature of bacterial growth. At this temperature, lysozyme functions efficiently to lyse bacterial cells, ensuring maximum yield of DNA extraction.
Upon addition to the isolation medium, lysozyme begins its work of breaking down the peptidoglycan layer of E. coli cell walls. By hydrolyzing the glycosidic bonds within peptidoglycan, lysozyme weakens the structural integrity of the cell wall, ultimately leading to cell lysis. This process releases the cellular contents, including plasmids, into the surrounding medium, where they can be further purified and isolated for downstream applications.
Conclusion:
In conclusion, lysozyme plays a critical role in plasmid isolation from E. coli by facilitating the lysis of bacterial cells. Through its enzymatic activity, lysozyme targets and disrupts the peptidoglycan layer of the bacterial cell wall, leading to cell lysis and the release of genetic material. Operating optimally at physiological temperatures, lysozyme ensures efficient extraction of plasmids, thereby enabling downstream molecular biology applications.