Plasmid Isolation 101: Why add Potassium acetate?

Introduction:
Potassium acetate serves as a crucial component in plasmid isolation protocols from Escherichia coli (E. coli), contributing to the efficient extraction of DNA. In this article, we'll explore the significance of potassium acetate in the isolation process, its function as a neutralizing agent, and its role in precipitating lipids and proteins from the medium.
Neutralizing Agent and Precipitation of Lipids and Proteins:
Potassium acetate acts as a neutralizing agent in the plasmid isolation process, helping to counteract the effects of previously added reagents such as sodium hydroxide (NaOH) or sodium dodecyl sulfate (SDS). By adjusting the pH of the solution, potassium acetate neutralizes any residual strong base present from the denaturation step, ensuring that the solution returns to a near-neutral pH suitable for subsequent steps.
Furthermore, potassium acetate induces the precipitation of lipids and proteins present in the medium. The addition of potassium acetate causes the salts of fatty acids and proteins to precipitate out of solution, forming visible aggregates. This precipitation step is essential for removing contaminants that could interfere with the isolation and purification of plasmid DNA.
Formation of KDS Precipitate:
Another notable effect of potassium acetate addition is the formation of potassium dodecyl sulfate (KDS) precipitate. SDS, added earlier in the isolation process as a detergent to lyse bacterial cells, reacts with potassium ions from potassium acetate to form insoluble KDS precipitate. This reaction helps to sequester SDS along with any associated lipids and proteins, further aiding in their removal from the solution.
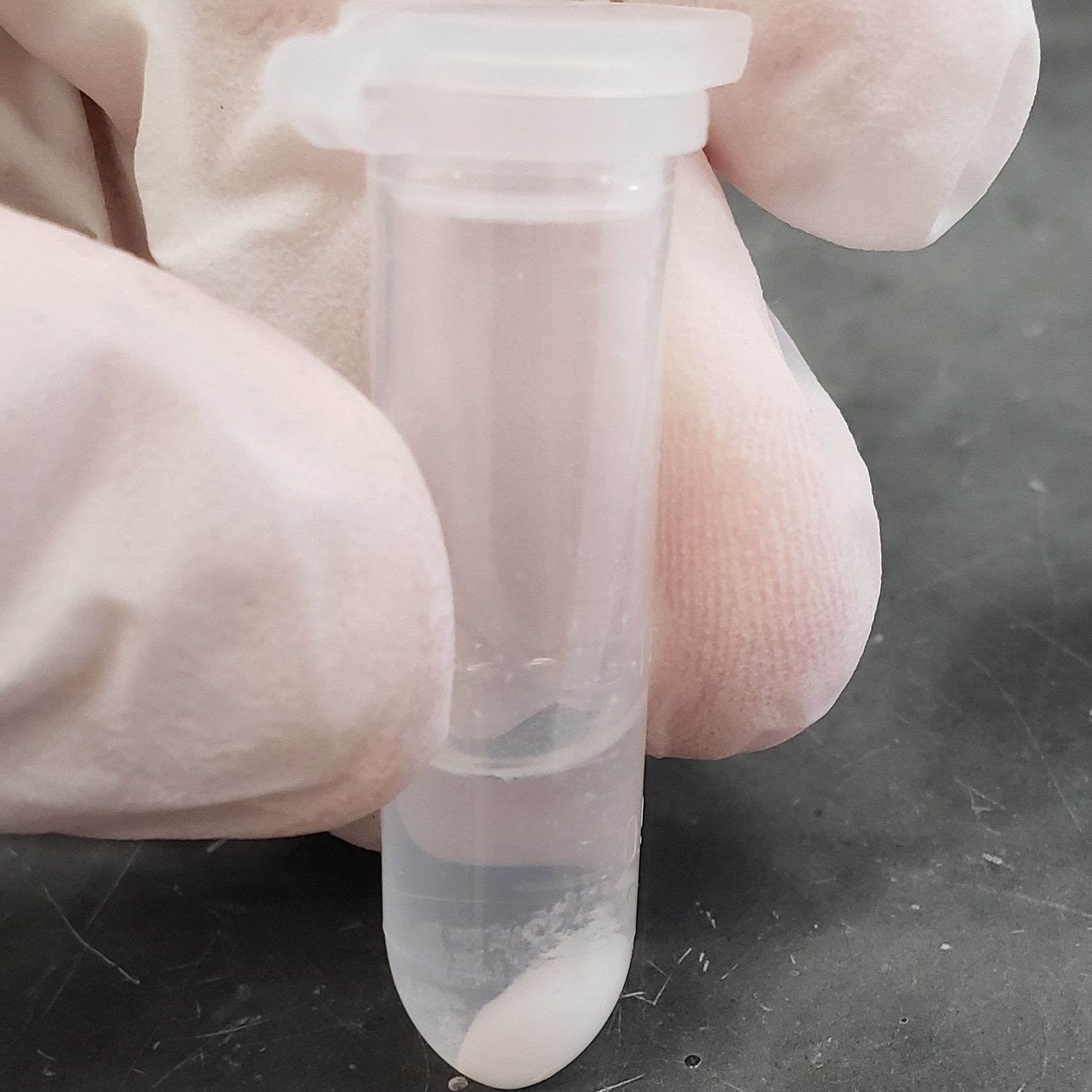
Centrifugation at Low Temperature:
Following the addition of potassium acetate and the precipitation of contaminants, the solution undergoes centrifugation to separate the precipitated material from the desired DNA. Centrifugation is typically conducted at 4° C to prevent heat damage to the DNA molecules. Operating at low temperatures helps maintain the stability of DNA, ensuring its integrity throughout the isolation process.
Conclusion:
In conclusion, potassium acetate plays a multifaceted role in plasmid isolation from E. coli, serving as a neutralizing agent, inducing precipitation of lipids and proteins, and facilitating the removal of contaminants through subsequent centrifugation. Its ability to neutralize strong bases, precipitate unwanted molecules, and aid in the purification of DNA underscores its importance in achieving successful plasmid isolation. By understanding the role of potassium acetate in the isolation process, researchers can optimize their protocols for efficient extraction of high-quality plasmid DNA for downstream applications in molecular biology.